Sustainable Governance
Drug Safety
Center Ventures Group corporate values include “Innovation, Integrity, Professionalism, Common Goods.” We enforced stringent manufacturing practices and quality control standard for all of our products starting from research and development, mass production and product sales. We safeguarded our customers health by providing health relating products and services with effective drugs. Prior to drug manufacturing licenses approval, our group constantly focused on raw materials and its formulation impurities research and carryout stress test when needed and to conduct all kinds of analysis and chemical examination to ensure the product quality and stability. All of our products needed to manufacture in the PIC/S standard plant and produce at least three batches to ensure its quality. These qualified products can then be use in clinical trials and to apply for drug manufacturing licenses approval.
All of our product sales are required to sell and procure by medical clinics, pharmacy, and hospitals channels. In addition, to ensure the best treatment and to protect patients’ privacy, all drugs will be prescribed by doctors. Our group also offers customer services and professional pharmacists to help on any inquiries on any medical drug usages. Taiwan Food & Drugs Administrations also established an “adverse drug reactions” reporting system that deals with patients on any adverse drug reactions report. Center Ventures Group also setup a “Drug Relief Foundation” that specializing in helping patients with any emergency relief caused by adverse drug reactions.

Supply Chain Management
As a responsible enterprise, our group hope to create a long-term, mutual trust and mutual benefits cooperations with supply chain. Our group works diligently to integrate the ESG ethos into our daily operations with the aim of sustainable development, setting systematic management strategies, taking tangible action, and reviewing results. We continue to work closely with partners to ensure that they meet our sustainability development requirements and share the same ESG values. We encouraged our supply chain to promote industry dialogue and development, as well as track key issues such as environmental sustainability, more humanistic, and transparent way to create a common good in our communities.
Supply Chain Assessment (Three Ratings)
| Ratings | Standard Requirements |
| A | Qualify International or domestic GMP or ISO certified suppliers that continue to offer stable quality raw materials for two consecutive years or more than 3 batches. |
| B | Qualify International or domestic GMP or ISO certified suppliers that continue to offer stable quality raw materials for less than two consecutive years or fewer than 3 batches. |
| C | New suppliers or re-evaluate suppliers |
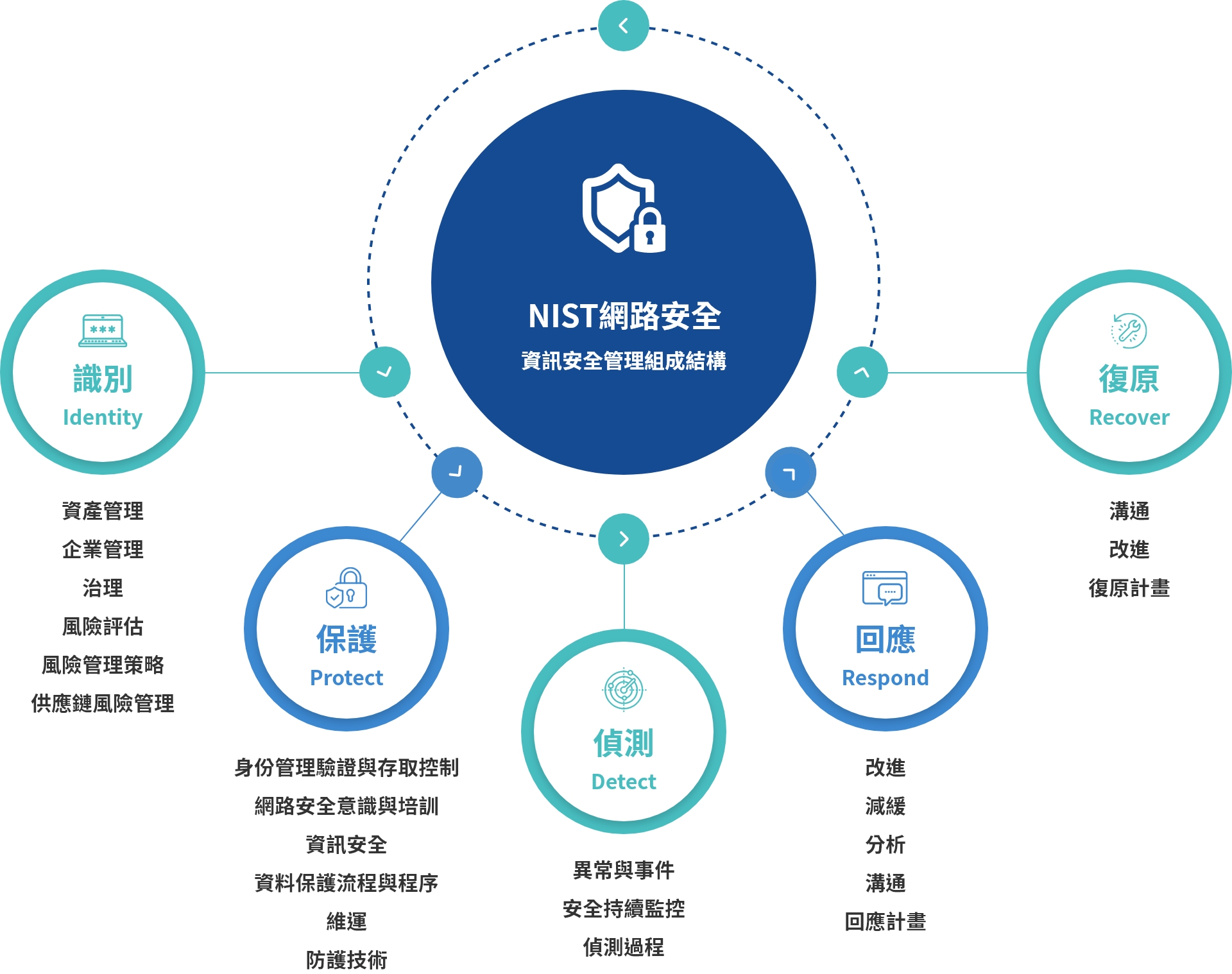
Information Security
Center Ventures Group is committed to ensure information security of customers and products. Our group continue to introduce comprehensive information security management mechanisms to ensure normal business operations and lowering the information security risks. We regularly implements internal exercises and training for information security to increase employees' information security awareness and vigilance while ensuring customer and product information security.